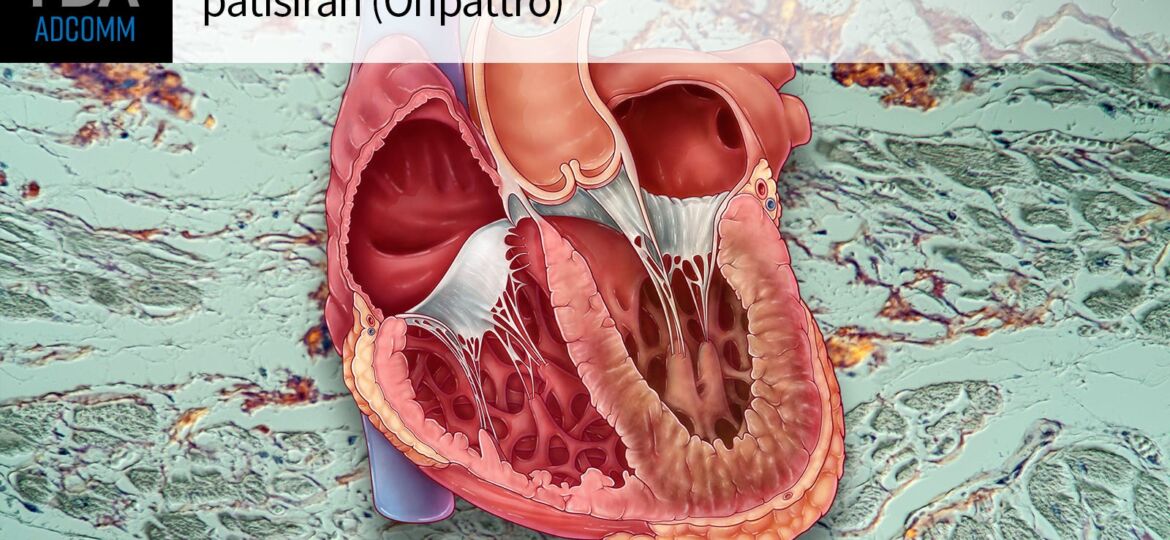
MedPage Today) — FDA advisors conceded on Wednesday that the benefit-risk balance of patisiran (Onpattro) technically checks out in wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM, also known as cardiac ATTR amyloidosis…
Read More

